| Symbol/Statement | Standard/Symbol Reference Number | Title of Symbol | Description of Symbol |
 |
ISO 15223-1, Clause 5.1.6 |
Catalog number | Indicates the manufacturer’s catalog number so that the medical device can be identified. |
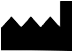 |
ISO 15223-1, Clause 5.1.1 |
Manufacturer | Indicates the medical device manufacturer. |
 |
ISO 15223-1, Clause 5.1.5 |
Batch code | Indicates the manufacturer’s batch code so that the batch or lot can be identified. |
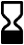 |
ISO 15223-1, Clause 5.1.4 |
Use by date | Indicates the date after which the medical device is not to be used. |
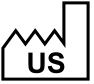 |
ISO 15223-1, Clause 5.1.11 |
Country of manufacture (with date of manufacture adjacent) | Identifies the country of manufacture of products and indicates the date when the medical device was manufactured. |
 |
IEC 60601-1, Table D.2, Symbol 2 | General warning sign | Indicates that caution is necessary when operating the device; the current situation needs operator awareness or operator action in order to avoid undesirable consequences. |
 |
IEC 60601-1, Table D.2, Symbol 10 | Refer to instruction manual/booklet | To signify that the instruction manual/booklet must be read |
 |
ISO 15223-1, Clause 5.4.3 |
Consult Instructions for Use | Indicates the need for the user to consult the instructions for use. |
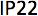 |
IEC 60529 / Degrees of protection provided by enclosures (IP Code) | n/a | Protected against fingers or objects › 12.55 mm and water spray less than 15° from the vertical. |
 |
ISO 15223-1, Clause 5.2.8 |
Do not use if package is damaged | Indicates a medical device that should not be used if the package has been damaged or opened. |
 |
ISO 15223-1, Clause 5.4.2 |
Do not reuse | Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure. |
 |
ISO 15223-1, Clause 5.2.6 |
Do not resterilize | Indicates a medical device that is not to be resterilized. |
 |
ISO 15223-1, Clause 5.2.3 |
Sterilized by ethylene oxide | Indicates a medical device that has been sterilized using ethylene oxide. |
 |
ISO 15223-1, Clause 5.2.11 |
Single sterile barrier system | Indicates a single sterile barrier system |
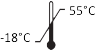 |
ISO 15223-1, Clause 5.3.7 |
Temperature limit | Indicates the temperature limits to which the medical device can be safely exposed. |
 |
IEC 60417, Reference No. 5840 |
Type B Applied Part | To identify a type B applied part complying with IEC 60601-1. |
| ISO 15223-1, Clause 5.7.7 | Medical device | Indicates the item is a medical device | |
 |
ISO 15223-1, Clause 5.1.2 | Authorized representative in the European Community | Indicates the authorized representative in the European Community. |
 |
Medical Device Directive 93/42/EEC, Medical Device Regulation 2017/745 | CE marking | ‘CE marking’ means a marking by which a manufacturer indicates that a device is in conformity with MDD 93/42/EEC or MDR 2017/745 and other applicable Union harmonisation legislation |
| ISO 15223-1, Clause 5.1.8 | Importer | Indicates the entity importing the medical device into the locale | |
 |
21 CFR 801.109 | Prescription only | Requires a prescription in the United States. |
| UK MDR 2002 | UK Conformity Assessed Marking | Indicates that a device is in conformity with UK MDR 2002 | |
 |
ISO 15223-1, Clause 5.2.7 | Non-sterile | Indicates a medical device that has not been subjected to a sterilization process. |
| Not made with natural rubber latex | FDA Guidance, December 2, 2014 | – | Indicates the product is not made with natural rubber latex |