Heavy menstrual bleeding (HMB) affects over 10 million women in the United States with more than 2 million women seeking care for the condition annually. Endometrial ablation is widely accepted throughout the developed world as filling an important gap between medical therapy and hysterectomy for treating HMB.1
Endometrial ablation is generally accepted to be a safe procedure that requires nominal training to perform and has a track record of substantial success with reduction of bleeding and improved quality of life.1 Compared to hysterectomy, endometrial ablation is less invasive, has fewer complications, has shorter procedure and recovery time, is less expensive, and has comparable short-term patient satisfaction rates.2
Even with all of these benefits, there remains a significant concern for both physicians and patients – the risk of postablative intracavitary scarring and adhesions which can lead to restricted access to the cavity post-ablation.
Here we will discuss why preserving cavity access after an endometrial ablation is so important, which factors contribute to limited access post-procedure, and how the different types of treatments available impact cavity access.
The importance of physicians being able to evaluate the uterine cavity post-ablation cannot be overstated. In fact, maintaining post-ablation cavity access is a key clinical outcome of endometrial ablation and is a commonly expressed concern for many healthcare providers.3
This is because it is so crucial for physicians to have the ability to properly diagnose any post-ablation symptoms or complications in the future. For example, a patient suffering from abnormal uterine bleeding needs to be properly evaluated in order to determine the source of the bleeding. In this instance, it could be persistent bleeding that continues after ablation or it could be a sign of a much more serious condition like endometrial cancer. Because the symptoms of both these conditions can often be similar, a physician would have no way to distinguish without being able to visualize and assess the uterine cavity.
As with any type of endometrial ablation procedure, there is always a risk of some postablative intracavitary scarring and adhesions. However, a significant amount of scarring and contracture can lead to multiple long-term complications, including cyclic pelvic pain (CPP), delayed diagnosis of endometrial cancer, and postablation tubal sterilization syndrome (PATSS).
The combination of endometrial scarring and a bleeding source, such as functioning or regenerative endometrial tissue that was missed during endometrial ablation, often causes cyclic pelvic pain. Women who have experienced CPP have described this pain as sharp, stabbing, and labor-like.1
The delayed diagnosis of endometrial cancer is another complication of post-ablation intrauterine scarring.2 This is because extensive scarring in the endometrium makes it very difficult, and at times impossible, to perform a diagnostic hysteroscopy.4 In fact, a 2009 study found that endometrial biopsy failed in 23% of women with a history of thermal endometrial ablation.5
If this scarring obstructs bleeding from any remaining or regenerating endometrium, it can also cause a complication known as postablation tubal sterilization syndrome (PATSS) which occurs in women with previous tubal sterilization. It presents as CPP which is thought to come from trapped endometrial tissue within the uterine cornua that cannot pass retrograde through the distal fallopian tube causing cyclic swelling and pain.1 PATSS often manifests 6 to 10 months after treatment and can lead to more invasive surgical procedures, including hysterectomy.2 PATSS has been found to occur in up to 10% of patients post endometrial ablation.6 However, recent studies of the use of cryotherapy for endometrial ablation found that of the 97 subjects who had previous tubal ligation, none reported the signs and symptoms associated with PATSS.3
These complications and delayed diagnosis can also lead to higher risk of hysterectomy post-ablation because it restricts the physician’s ability to properly evaluate – and therefore treat – the patient. If the uterine cavity is inaccessible due to scarring or if no definitive diagnosis can be made, the patient may need a hysterectomy.4
This is of such concern that 87% of gynecologists indicated they would be more motivated to recommend endometrial ablation if uterine scarring were minimized.7
Hyperthermic (Heat-Based) Strategies
Radiofrequency ablation (RFA) comprises the majority of endometrial ablations (73%) performed annually. One problem with RFA is that it has been associated with a high risk of postablation uterine synechiae (adhesions) which can lead to further complications down the line.
The intrauterine scarring that occurs as part of the healing process with thermal ablations can also severely limit a physician’s ability to evaluate the endometrium with hysteroscopy or biopsy in the presence of abnormal uterine bleeding. This limitation is a serious concern for both physicians and patients because determining the source of abnormal bleeding is critical to diagnosing and treating abnormal uterine bleeding.3
Earlier studies on thermal ablation have also shown a greater risk of heat extending beyond the established area of treatment when using heat-based technologies. This means use of heat generating electrosurgical methods for endometrial ablation could potentially cause excessive scarring due to heat extending beyond the targeted treatment area.
Hypothermic (Cold-Based) Strategies3
Cryoanalgesia technology has continued to advance since the inception of cryoprobes in 1961. Freezing is an energy-deprivation strategy that causes ice formation and hypothermic stress to cells which ultimately leads to cell death.
Prior studies have shown that cryoablation provides a comparatively better tissue healing response that can lessen the occurrence of significant intrauterine adhesions thereby preserving endometrial cavity access. Despite documentation of the well-established advantages of cryotherapy over heat based ablation modalities, cryotherapy remains underutilized.
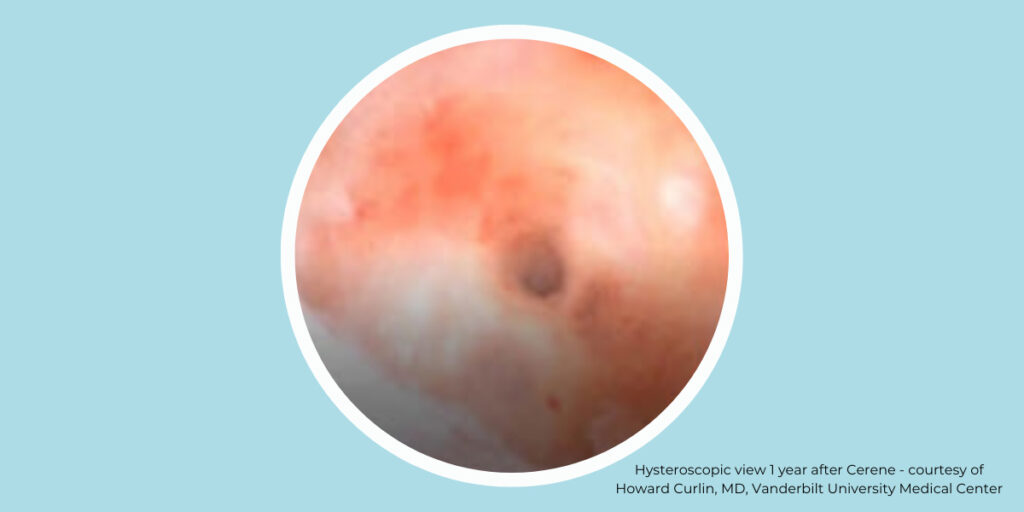
The Cerene Cryotherapy Device has shown that it can preserve access to the endometrial cavity demonstrated by hysteroscopy at 12 months post ablation and offers a safe alternative to hysterectomy, resectoscopic endometrial ablation (REA), and non-resectoscopic endometrial ablation (NREA). Cerene’s unique cryotherapy treatment provides an in-office treatment without the need for general anesthesia with a reduced occurrence of post ablation intrauterine synechiae.
Cryoablation procedures offer minimal risk of postablation intrauterine synechiae. This can provide the physician the ability to easily access the uterine cavity postablation for future diagnostic evaluation.
The CLARITY Study (2020) demonstrated the unique and clinically impactful outcome of minimized intrauterine synechiae.3 It is the first study to document the evaluation of the uterine cavity 12 months after cryoablation therapy.2
Results from The CLARITY Study for adequate visualization of the endometrial cavity after endometrial ablation with the Cerene Cryotherapy Device are below.
| Investigator Evaluation of Uterine Cavity | |
| Assessment (N=223) | Yes (%) |
| Uterine cavity entry with a hysteroscope | 220 (98.7%) |
| Full visualization of the uterine cavity | 204 (91%) |
| Investigator Assessment of Cavity Findings | |
| Assessment (N=204)* | Yes (%) |
| Overall, was the investigator satisfied that she/she was able to adequately visualize the endometrium to evaluate the uterine cavity for pathologic change? | 195 (95.6%) |
| Would the investigator be able to direct a biopsy anywhere within the uterine cavity? | 178 (87.3%) |
| *Uterine cavities that could be fully visualized | |
| The CLARITY Study Results | |
| Source: Cerene Physician Labeling Document |
As demonstrated by the results of The CLARITY Study, cryoablation is a well-characterized therapy which, when used in the uterus, reduces the likelihood of scarring and adhesion. At 12 months, investigators in the CLARITY Study were able to fully visualize the endometrium to evaluate the uterine cavity for pathologic changes in 96% of subjects with accessible uterine cavities.2
The Cerene Cryotherapy Device offers a consistently safe and effective in-office treatment for women choosing endometrial ablation for menorrhagia. Patient satisfaction was high for treatment effectiveness, minimal pain during and after the procedure, and post-ablation cavity access.
References:
Important Safety Information
Cerene® Cryotherapy Device is indicated to ablate the endometrial lining of the uterus in premenopausal women with heavy menstrual bleeding due to benign causes for whom childbearing is complete. Pregnancy following the Cerene procedure can be dangerous; therefore, contraception must be used until menopause. The Cerene procedure is not for those who have or suspect uterine cancer; have an active genital, urinary or pelvic infection; or an IUD. There are risks and considerations associated with the use of the Cerene Cryotherapy Device. Temporary side effects may include uterine cramping, vaginal infection, and lightheadedness. For detailed benefit and risk information, consult the Cerene Instructions for Use (IFU) or your healthcare professional. Learn more >
Important Safety Information
Cerene® Cryotherapy Device is indicated to ablate the endometrial lining of the uterus in premenopausal women with heavy menstrual bleeding due to benign causes for whom childbearing is complete. Pregnancy following the Cerene procedure can be dangerous; therefore, contraception must be used until menopause. The Cerene procedure is not for those who have or suspect uterine cancer; have an active genital, urinary or pelvic infection; or an IUD. As with all surgical procedures, there are risks and considerations associated with the use of the Cerene Cryotherapy Device. Temporary side effects may include cramping, nausea, vomiting, vaginal discharge and spotting. For detailed benefit and risk information, consult the Cerene Instructions for use (IFU) or your healthcare professional. Learn More
