
Endometrial ablation is a well-documented treatment for heavy menstrual bleeding (HMB) – also known as menorrhagia – offering long-term relief for patients grappling with excessive blood loss and dysmenorrhea.1 Yet, while addressing these symptoms is crucial, safety remains paramount for any OBGYN when considering endometrial ablation techniques.
Although endometrial ablation inherently presents fewer risks than more invasive menorrhagia treatments, such as hysterectomy, it’s essential to understand that no procedure is entirely without risk.2 Ablations, while effective, have the potential to affect surrounding structures, including the deep myometrium, bowel, or bladder. In response to these concerns, experts in the medical community should actively assess the safety features of various ablation techniques.
Endometrial Cryoablation With Cerene®
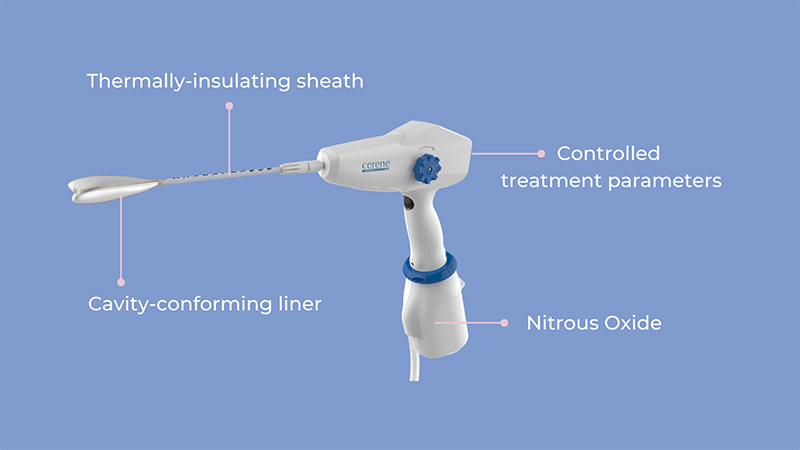
The Cerene Cryotherapy Device not only deploys cryothermal energy (in the form of nitrous oxide) into a cavity-conforming liner to ablate the endometrial lining, but also triggers a healing process unique to cryotherapy that results in minimal intrauterine adhesions. The cooling technology freezes the sensory nerves of the uterus, delivering a natural cryoanalgesic effect that reduces the need for general anesthesia or IV sedation for pain management. Cerene is designed with built-in safety features to maintain patient safety throughout the ablation procedure.
90% of patients are satisfied with Cerene:†
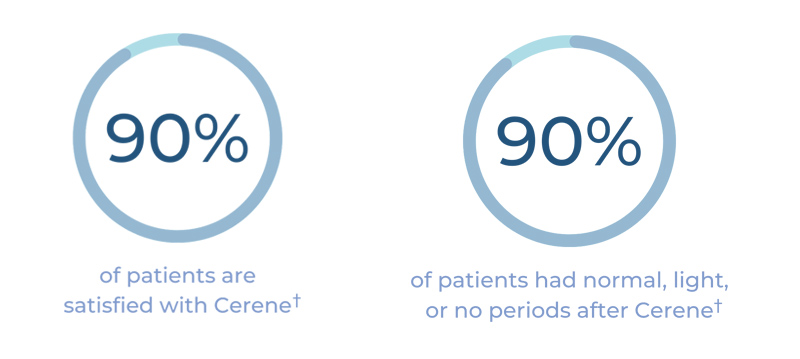
†Patient-reported data include definitely and consider recommending Cerene and are 1 year after treatment with durable results at 3 years.
How Cerene Promotes Patient Safety
The first step in driving treatment success and safety is selecting the right patient. Not every patient is a suitable candidate for endometrial ablation with the Cerene Device. Gynecologists must ensure their patient is not pregnant or planning future pregnancies, does not have known or suspected uterine cancer or unresolved endometrial hyperplasia, lacks certain anatomic or pathologic conditions that weaken the myometrium, has no history of prior endometrial ablation or resection, is free from active genital or urinary tract infections, does not have an IUD in place, and is not experiencing undiagnosed vaginal bleeding.4
Once patient eligibility is verified, OBGYNs can move forward with the cryoablation procedure, utilizing Cerene. From start to finish, the Cerene device prioritizes patient safety with a variety of multi-tiered features, including:
See Cerene in Action
Cerene takes cryoablation one step further with next-generation safety features that help prevent burns and prioritize patient safety, with no device- or procedure-related serious adverse effects reported in the pivotal clinical study.3 Gynecologists who perform endometrial ablation with Cerene can help their patients realize long-term clinical benefits by delivering a safe, well-tolerated, and effective treatment for HMB.
Learn more about how Cerene maintains patient safety during endometrial ablation.
† Patient-reported data are 1 year after treatment with durable results at 3 years
Key Takeaways:
Sources:
Important Safety Information
Cerene® Cryotherapy Device is indicated to ablate the endometrial lining of the uterus in premenopausal women with heavy menstrual bleeding due to benign causes for whom childbearing is complete. Pregnancy following the Cerene procedure can be dangerous; therefore, contraception must be used until menopause. The Cerene procedure is not for those who have or suspect uterine cancer; have an active genital, urinary or pelvic infection; or an IUD. As with all surgical procedures, there are risks and considerations associated with the use of the Cerene Cryotherapy Device. Temporary side effects may include cramping, nausea, vomiting, vaginal discharge and spotting. For detailed benefit and risk information, consult the Cerene Instructions for use (IFU) or your healthcare professional. Learn More
