Lasting Relief from Heavy, Painful Periods
Cerene® Endometrial Cryotherapy, a quick 2 1/2 minute treatment done right in your doctor’s office.
Doubling up on pads?
✳︎
Bleeding through clothes?
✳︎
Period longer than 7 days?
✳︎
Missing work?
Doubling up on pads? ✳︎ Bleeding through clothes? ✳︎ Period longer than 7 days? ✳︎ Missing work?
1 in 5 women suffer from heavy menstrual bleeding.¹
With bleeding that soaks through menstrual products for hours or days in a row, it can feel impossible to keep up with all of your normal daily activities – always worrying about having a bathroom nearby.
Introducing Cerene® Cryotherapy
Cerene is a treatment designed to provide relief from your heavy and painful periods, getting you back to a life controlled by you, not your period.
2 1/2 minute treatment time using cooling technology
No anesthesia needed
No incisions
Done right in your doctor’s office
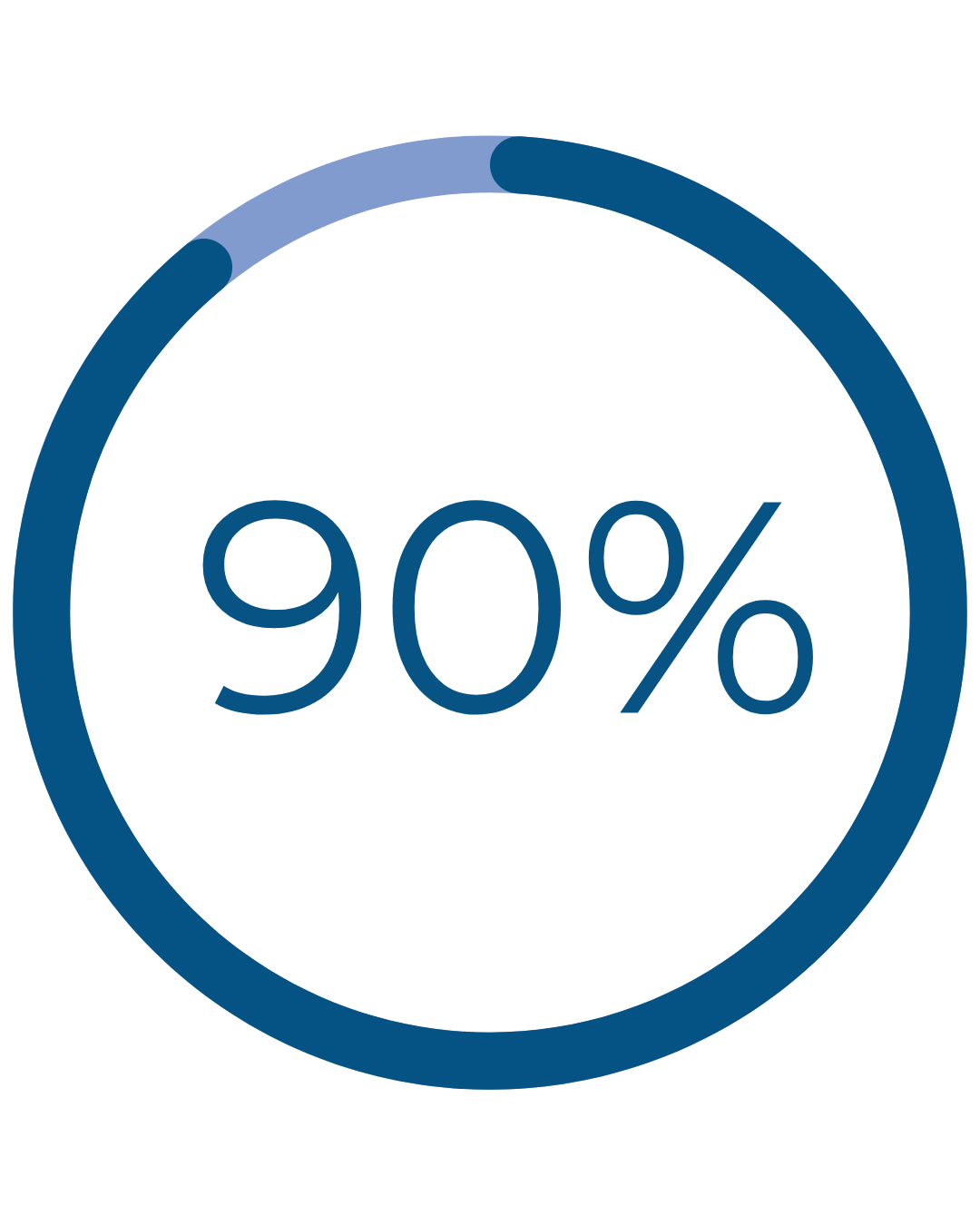
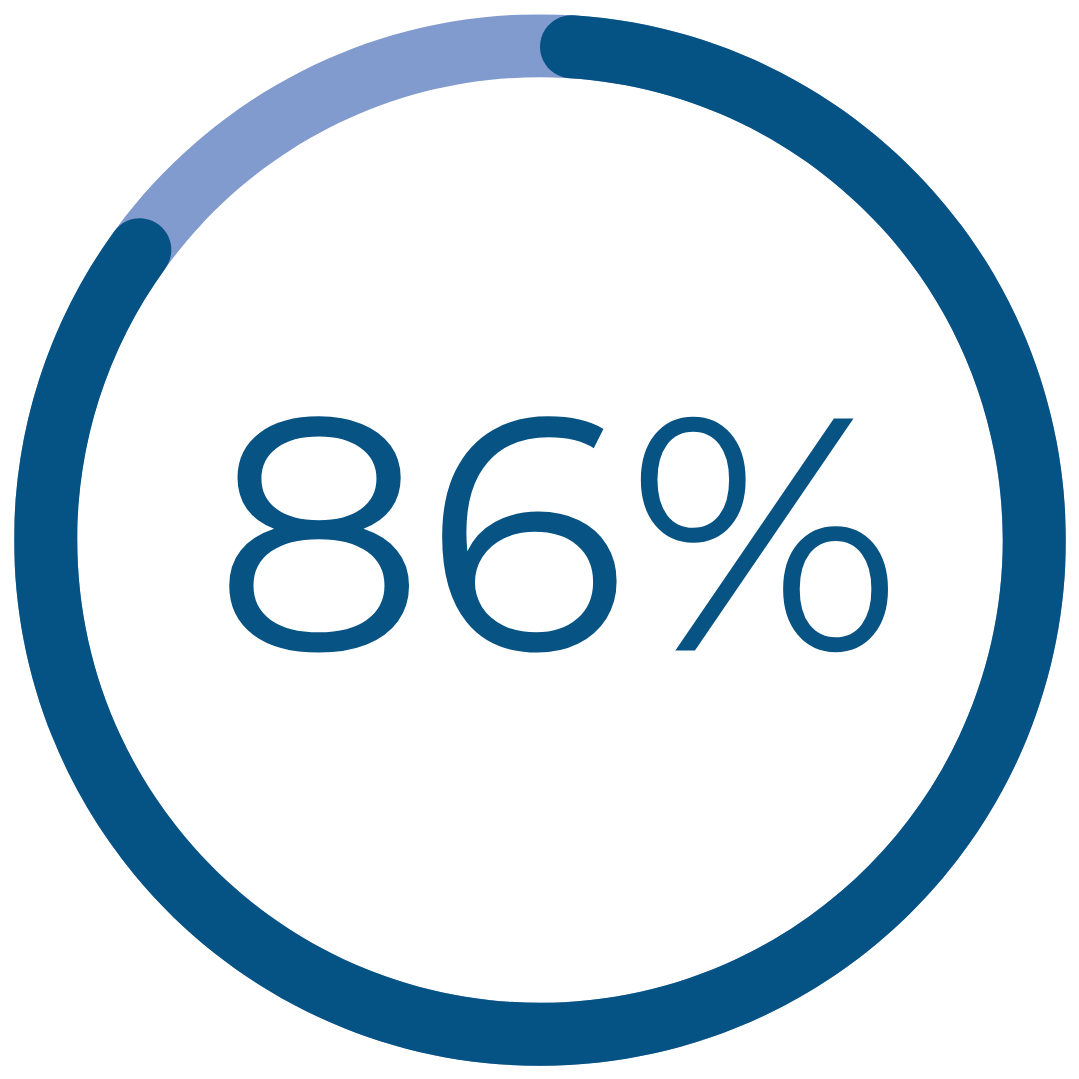
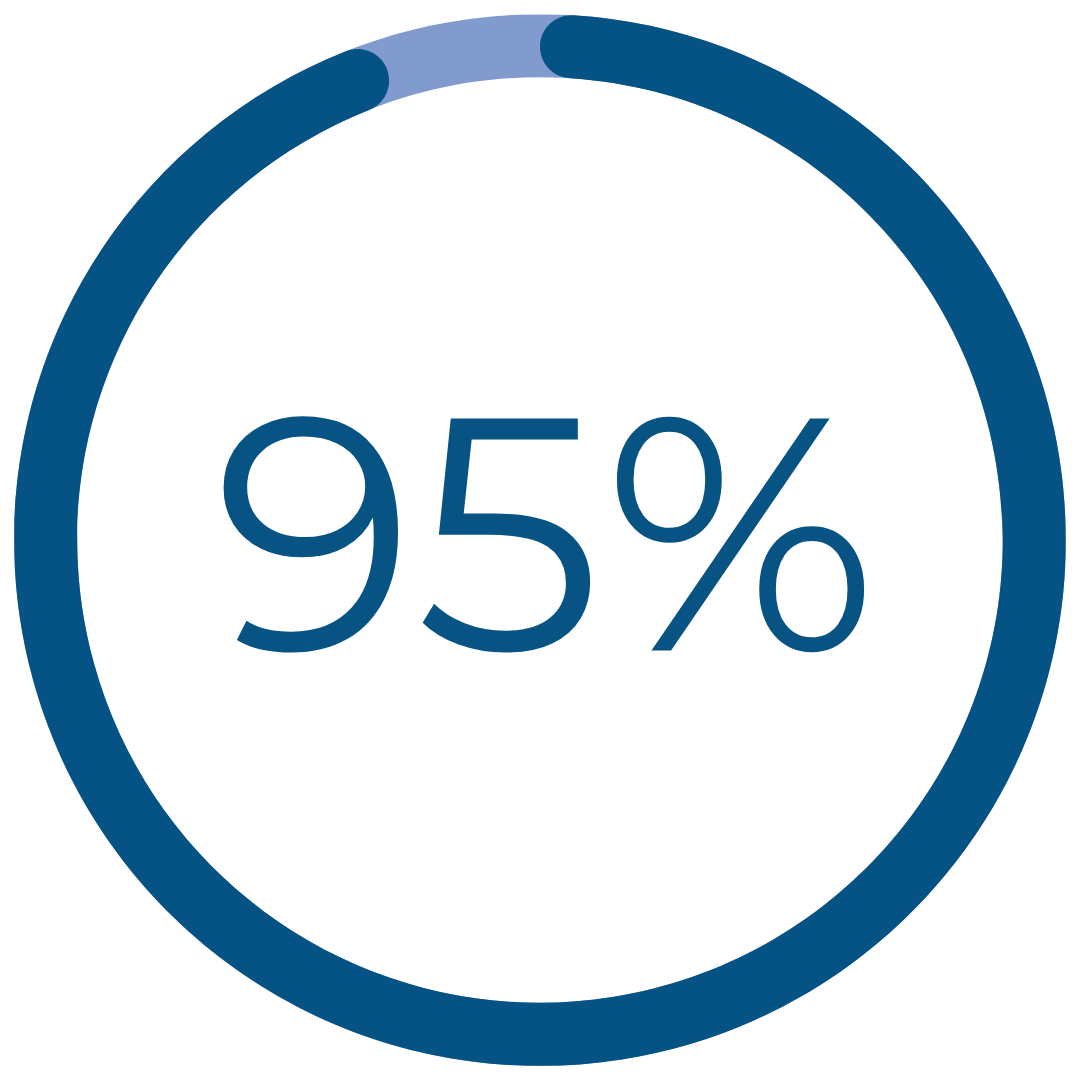
Real Patient Results
reported normal, light, or no periods after Cerene†
had a reduction in cramping‡
would recommend Cerene to family and friends†††
† Patient-reported data are 1 year after treatment with durable results at 3 years
‡ Improvement reported one year after treatment for patients reporting severe / very severe cramping
††† Patient-reported data include definitely and consider recommending Cerene and are 1 year after treatment with durable results at 3 years